Iloprost IV has been proven effective for patients with severe frostbite
Effectiveness was established in young, healthy
adults who suffered frostbite at high altitudes.1


Iloprost IV significantly decreased the risk of digit amputation in patients with severe frostbite1
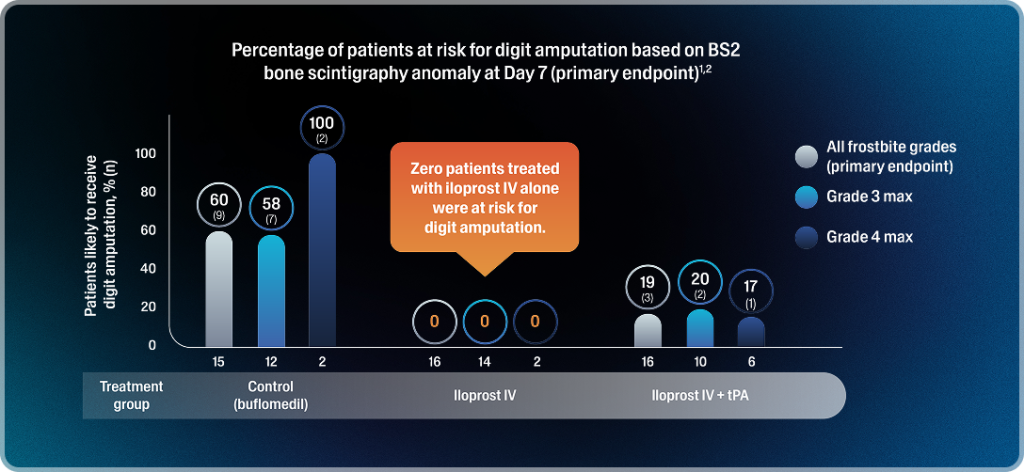
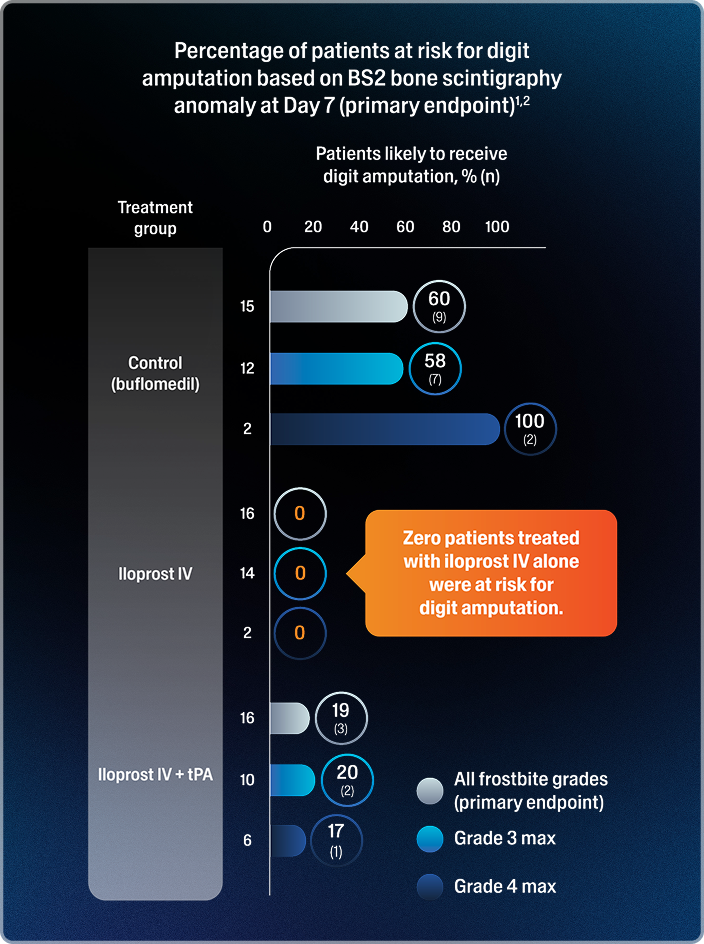
- In the iloprost IV + tPA group, the risk of digit amputation was 19%, and 3% (5/159) of digits were likely to be amputated1,2,*
- In the control group, the risk of digit amputation was 60%, and 40% (42/106) of digits were likely to be amputated1,2,*
Among the 40 patients with available 3-month follow-up data, amputation outcomes were correctly predicted in all cases.1,2,†
*The secondary endpoint was the percentage of digits at risk of amputation due to frostbite based on BS2 bone scintigraphy anomaly.2
†Three-month post-treatment data were unavailable for 7 of the 47 patients; among them, 2 were predicted to require amputation (1 in the control group and 1 in the iloprost IV + tPA group).1,2
IV=intravenous; tPA=tissue plasminogen activator.
Iloprost IV was studied and proven effective in an open-label, randomized, controlled study of patients with severe frostbite1
- Severe frostbite was defined as having at least one digit (finger or toe) with frostbite Grade 3 (lesion extending just past the proximal phalanx) or Grade 4 (lesion extending proximal to the metacarpal or metatarsal joint)
- The mean age of the study population (N=47) was 33 years (range: 18-55 years), 94% of patients were men, and 96% sustained frostbite at high altitudes
- At baseline, 70% of enrolled patients had frostbite involving the feet, 62% had frostbite involving the hands, and 32% had frostbite involving both feet and hands
All eligible patients were treated with rapid rewarming of areas with frostbite, aspirin 250 mg IV, and buflomedil 400 mg IV and then randomized into 3 groups1:

received buflomedil 400 mg IV for up to 8 days
received iloprost IV for 6 hours daily for up to 8 days
received recombinant tPA IV on Day 1 and iloprost IV for 6 hours daily for up to 8 days
All patients continued to receive aspirin 250 mg IV daily for up to 8 days.
- The primary endpoint was the presence of an anomaly on a bone scintigraphy performed 7 days after initial clinical presentation of frostbite in at least one finger/toe affected by severe frostbite. The bone scintigraphy anomaly is expected to predict risk of amputation1
- Results shown are based on patients receiving iloprost IV within 48 hours after rewarming2
Demonstrated safety in clinical trials
Explore the safety and tolerability profile of iloprost IV.1-4
INDICATIONS AND USAGE
AURLUMYN is a prostacyclin mimetic indicated for the treatment of severe frostbite in adults to reduce the risk of digit amputations. Effectiveness was established in young, healthy adults who suffered frostbite at high altitudes.
IMPORTANT SAFETY INFORMATION
Warnings and Precautions
AURLUMYN may cause symptomatic hypotension. Correct hypotension prior to administration of AURLUMYN. Monitor vital signs while administering AURLUMYN.
Adverse Reactions
Adverse events reported with the use of intravenous (IV) iloprost in patients with frostbite include headache, flushing, palpitations/tachycardia, nausea, vomiting, dizziness, and hypotension.
Use in Specific Populations
- Advise women not to breastfeed during treatment with AURLUMYN.
- The safety and efficacy of AURLUMYN in pediatric patients have not been established.
- Dosage adjustment is recommended in patients with moderate or severe hepatic impairment.
- In patients with eGFR <30 mL/min, dosage adjustment can be considered based on tolerability. The effect of dialysis on the clearance of AURLUMYN has not been evaluated.
To report suspected adverse reactions, contact BTG at 1-877-377-3784 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see full Prescribing Information.
- AURLUMYN (iloprost) [prescribing information]. BTG International, Inc.
- Cauchy E, Cheguillaume B, Chetaille E. A controlled trial of a prostacyclin and rt-PA in the treatment of severe frostbite. N Engl J Med. 2011;364(2):189-190; Cheguillaume B. Controlled trial of iloprost and iloprost and rt-PA in the treatment of severe frostbite. Doctoral thesis. Grenoble School of Medicine; 2011.
- Krause W, Krais T. Pharmacokinetics and pharmacodynamics of the prostacyclin analogue iloprost in man. Eur J Clin Pharmacol. 1986;30(1):61-68.
- Krause W, Krais T. Pharmacokinetics and pharmacodynamics of radio-labeled iloprost in elderly volunteers. Eur J Clin Pharmacol. 1987;32(6):597-605.



