AURLUMYN is the first and only FDA-approved treatment for severe frostbite and has a dual mechanism of action
FDA=US Food and Drug Administration.


In severe frostbite, direct and indirect injury lead to cell death1-3
- Low temperatures result in tissue cooling, vasoconstriction, and decreased blood flow in extremities
- Prolonged decreased blood flow and low temperatures lead to the formation of ice crystals in the blood and subsequent changes in cell structure (direct injury). As tissues rewarm, the ice crystals begin to melt, causing tissue edema and inflammation (indirect injury)
- While the direct injury has already occurred, indirect injury after rewarming remains an opportunity for intervention and improved outcomes
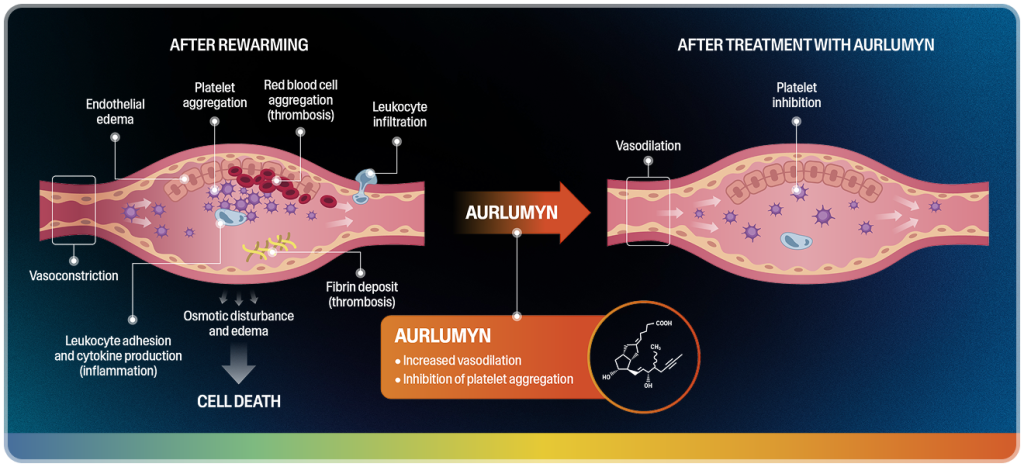
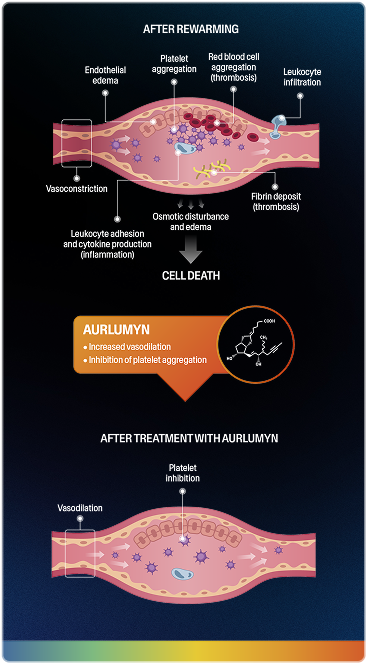
AURLUMYN is a synthetic analog of prostacyclin, a naturally occurring molecule in the body2,4-7
AURLUMYN rapidly and simultaneously dilates blood vessels and inhibits platelet aggregation—2 key components of secondary injury in frostbite.
Together, these actions interrupt the secondary injury cascade and reduce the risk of digit amputation.
AURLUMYN has a short half-life of 20 to 30 minutes,
which allows for a rapid and titratable effect.4-7
Puts digit preservation within reach
AURLUMYN is the only FDA-approved treatment for severe frostbite in adults to reduce the risk of digit amputations.4
INDICATIONS AND USAGE
AURLUMYN is a prostacyclin mimetic indicated for the treatment of severe frostbite in adults to reduce the risk of digit amputations. Effectiveness was established in young, healthy adults who suffered frostbite at high altitudes.
IMPORTANT SAFETY INFORMATION
Warnings and Precautions
AURLUMYN may cause symptomatic hypotension. Correct hypotension prior to administration of AURLUMYN. Monitor vital signs while administering AURLUMYN.
Adverse Reactions
Adverse events reported with the use of intravenous (IV) iloprost in patients with frostbite include headache, flushing, palpitations/tachycardia, nausea, vomiting, dizziness, and hypotension.
Use in Specific Populations
- Advise women not to breastfeed during treatment with AURLUMYN.
- The safety and efficacy of AURLUMYN in pediatric patients have not been established.
- Dosage adjustment is recommended in patients with moderate or severe hepatic impairment.
- In patients with eGFR <30 mL/min, dosage adjustment can be considered based on tolerability. The effect of dialysis on the clearance of AURLUMYN has not been evaluated.
To report suspected adverse reactions, contact BTG at 1-877-377-3784 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see full Prescribing Information.
- Fudge J. Exercise in the cold: preventing and managing hypothermia and frostbite injury. Sports Health. 2016;8(2):133-139.
- Knapik JJ, Reynolds KL, Castellani JW. Frostbite: pathophysiology, epidemiology, diagnosis, treatment, and prevention. J Spec Oper Med. 2020;20(4):123-135.
- Hickey S, Whitson A, Jones L, et al. Guidelines for thrombolytic therapy for frostbite. J Burn Care Res. 2020;41(1):176-183.
- AURLUMYN (iloprost) [prescribing information]. BTG International, Inc.
- Straaten HMOV. Anticoagulation strategies for continuous renal replacement therapy. In: Ronco C, Bellomo R, Kellum JA, Ricci Z, eds. Critical Care Nephrology. 3rd ed. Elsevier, Inc; 2017:1018-1023.
- Krause W, Krais T. Pharmacokinetics and pharmacodynamics of the prostacyclin analogue iloprost in man. Eur J Clin Pharmacol. 1986;30(1):61-68.
- Krause W, Krais T. Pharmacokinetics and pharmacodynamics of radio-labeled iloprost in elderly volunteers. Eur J Clin Pharmacol. 1987;32(6):597-605.



